Posts Tagged ‘COVID19’
Pregnant or worried about infertility? Get vaccinated against COVID-19
Pregnant or worried about infertility? Get vaccinated against COVID-19
Matthew Woodruff, Emory University
As the delta variant of SARS-CoV-2 surges across the U.S., almost 1 in 5 Americans continue to resist getting shots that are widely available, safe and effective – particularly for preventing the most severe outcomes of the virus.
While people have many different justifications for not getting the shot, one particularly insidious bit of pseudoscience has surfaced. It is routinely invoked in the contentious debate over vaccine policy in the U.S. and continues to stir confusion and skepticism toward vaccines in young women across the globe.
This misinformed argument reasons that the coronavirus vaccines could affect fertility in women by mistakenly triggering the creation of antibodies that react with an important placental protein called syncytin-1. This protein contains minor similarities to the coronavirus spike protein used in all current COVID-19 vaccines. Thus, the false narrative goes, the immune system will not be able to differentiate between the two and will create antibodies that interfere with proper development of the placenta.
This argument lacks understanding of how the immune system does its job.
As an immunologist who studies COVID-19 infection and the ways it can cause the immune system to turn against itself, this misunderstanding comes up frequently in my conversations with friends, family members and even medical workers who are legitimately concerned about their health and their future ability to have children.
It is completely understandable to have questions about how a new vaccine might affect reproductive health. But the science is clear that getting vaccinated does not put women at risk for infertility. It protects women, their unborn children and their families from a serious disease that, ironically, could in fact affect fertility in men.
Antibodies rarely make mistakes
The immune system is an immensely complicated network of cells, tissues and proteins that interact with one another – and the outside world. It works to maintain a balanced, healthy environment so the rest of the cells in the body can do their jobs. Among other things, the immune system helps direct fetal development, oversees and manages the microbes that aid in digestion and, of course, fights off infection.
One of the immune system’s most critical jobs is to differentiate between the body’s own cells and those of outside invaders to prevent accidental attacks on itself. In immunology, this careful selection of responses is called “immune tolerance.” People whose immune systems fail to maintain this tolerance and instead attack their own cells and tissues are diagnosed with autoimmune disorders. These can range in symptoms and severity depending on the tissue being attacked. An example is rheumatoid arthritis – a misdirected antibody attack on soft tissue in the joints.
The immune system has a series of checks and balances that are intended to prevent such autoimmune attacks. When B-cells – the cells in the immune system that produce antibodies – are first “born,” they carefully screen themselves to make sure that they won’t target the body’s own organs. That self-screening continues as B-cells patrol the body looking for an infection to fight; if they find something potentially threatening, like a vaccine, they engage in a highly orchestrated dance with other immune cells. Through that weeks-long process, only B-cells that produce antibodies against the outside invader survive. B-cells with self-destructive potential are killed.
Importantly, in parts of the body where it is absolutely critical that the immune system not mistakenly turn on its own cells – such as a developing placenta or in the brain – the entire region is immunosuppressive. This means that the threshold for activating the body’s immune response in those areas is set at an even higher bar.
This is not emerging science. These are well-established concepts among immunology experts. and have been for almost a half-century. As a result, it was not particularly noteworthy that a new preliminary study of women with fully developed immune responses against coronavirus showed no activity against the placental protein syncytin-1. Another study unsurprisingly demonstrated that the vaccine does not damage the placenta.
COVID-19 is the real threat to the immune system
It is important to remember that the COVID-19 vaccines authorized – and in the case of Pfizer-BioNTech, fully approved – in the U.S. carry the instructions to make the same spike protein that the virus uses to force its way into cells. Regardless of whether a person is infected with COVID-19 or receives a vaccine that emulates part of the virus, the immune system will respond aggressively to the spike protein that the body sees as foreign. Study after study confirm that in people who contract the virus, the majority of the immune response is directed at the spike protein.
However, there is one critical difference between vaccination and infection.
When you get vaccinated, your immune system has the time to respond under relatively low-risk circumstances. In other words, the immune system senses a threat and begins to build up its arsenal without rushing. But when it is confronted with a severe infection, the immune system recruits every weapon it has, as quickly as possible, to fend off severe infection or death.
[Understand new developments in science, health and technology each week. Subscribe to The Conversation’s science newsletter.]
This is important because we now know that under the severe stress of fighting COVID-19, the immune system fires up an emergency response pathway and begins producing antibodies that are not well selected. Many of these antibodies will target the virus, but our work now under review and others’ published findings confirm that in more than half of severe patients, a large number of antibodies also target their own cells.
Simply put: The danger of this kind of “auto-reactivity” in COVID-19 doesn’t come from responding against the spike protein in a vaccine – it occurs when the body has to fight a real COVID-19 infection.
Getting vaccinated protects unborn children
Getting vaccinated costs people a couple of days of not feeling 100%. In return, it provides protection from contracting a serious disease with the potential to cause serious illness or death. Being vaccinated also gives crossover protection to an unborn child.
COVID-19 infection, on the other hand, puts pregnant women at risk of severe disease, pregnancy complications and death. It may also affect a couple’s ability to have children by decreasing a man’s sperm counts and causing erectile dysfunction.
The science is clear, but for me this is also deeply personal. My wife was vaccinated in March, and we are expecting a baby in December. We are both deeply grateful for a vaccine that has given us the confidence to support a healthy pregnancy in the midst of a pandemic.
Matthew Woodruff, Instructor of Human Immunology, Emory University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Vaccines 101 – An immunologist’s perspective on vaccines and vaccine hesitancy
All about vaccines! Topics include history of vaccine hesitancy, immune responses to vaccines, types of vaccines, and vaccine safety. Brought to you by Jefferson’s Electorate.
Want more? Please consider donating!
Immune interference – why even ‘updated’ vaccines could struggle to keep up with emerging coronavirus strains
Immune interference – why even ‘updated’ vaccines could struggle to keep up with emerging coronavirus strains
Matthew Woodruff, Emory University
Despite the success and optimism of the new COVID-19 vaccination campaigns being rolled out worldwide, the emergence of new viral strains threatens to undermine their effectiveness. Indeed, South Africa has been forced to rethink its strategy as its initial vaccine of choice failed to provide protection to an emerging, but now dominant, viral variant.
Hope is still high that the mRNA-based vaccines licensed in the U.S., with their spectacular efficacy, will continue to provide protection despite impaired targeting of new strains. The jury is still out on viral vector vaccines, like the new Johnson & Johnson vaccine, but early data showing a reduced effectiveness against the South African variant has raised alarms.
RNA viruses, like coronaviruses, are known for their ability to mutate. With continued widespread infection, the opportunity for the virus to mutate and evade ongoing vaccination efforts remains high. Many in the scientific community have felt comfortable in the knowledge that mRNA-based vaccines can be quickly modified and redeployed. If the our current vaccines fail, we revaccinate individuals with obsolete immunity against the new strains, and play global whack-a-mole as the virus evolves.
But it may not be that easy.
As an immunologist who studies how antibody responses choose their targets, I am concerned that these “vaccine updates” may be less effective in patients that have already received their original shots. Immunological memory, the very thing that offers continued protection against a virus long after vaccination, can sometimes negatively interfere with the development of slightly updated immune responses. The scientific community needs to get ahead of this emerging problem and investigate vaccine approaches known to reduce the potential for viral escape. https://www.youtube.com/embed/XHd3CUYEO9o?wmode=transparent&start=0 Dr. Scott Gottlieb, former FDA commissioner, discusses coronavirus variants and adjusting to them.
Vaccines are designed to generate immune memory
In simplest terms, vaccines are a way to give your immune system a sneak peek at a pathogen. There are different ways to do this. One way is to inject inactivated versions of a virus, as has been done with polio. Another is to use noninfectious viral components such as the proteins used for flu vaccines. And most recently, scientists have found ways to deliver mRNA “instructions” that tell your body how to make those noninfectious viral components, as has been done with the Moderna and Pfizer vaccines against COVID-19. These vaccines all train your immune system to identify and respond against critical components of a potential invader. An important part of that response is to get your body to produce antibodies that will hopefully prevent future infections, breaking the cycle of person-to-person transmission.
However, it takes time for your immune system to generate those protective responses. Your immune system is immensely powerful – capable of destroying dangerous pathogens as well as your own tissue. The risk of accidentally producing antibodies that attack your own body is both very real, and potentially catastrophic.
To prevent this, your immune system rigorously tests immune cells that produce antibodies – called B cells – to make sure that they are responding with high specificity to the pathogen and not your own tissue. This process can take weeks. Rushing it carries risks, and may be an important component of the manifestations of severe COVID-19.
Vaccination gives your body the time to safely carry out that process – generating antibodies against the pathogen that pose no risk to your own cells. The antibodies you produce in that time will last months, and your immune system also remembers how to make them. The establishment of immune memory is a critical component of vaccines. The ability to remember what your immune system has responded against in the past gives it a significant edge when it encounters the same pathogen in the future.
But what happens when the virus evolves, and that memory becomes “obsolete”? https://www.youtube.com/embed/w4sUuFBEo2g?wmode=transparent&start=0 mRNA vaccines work differently than older vaccines.
The specter of ‘original antigenic sin’
During a response to a pathogen, such as a virus, your immune system produces large amounts of a limited set of antibodies. Think of a virus as a car trying to run you over. You might produce one kind of antibody against the hood, one against the bumper, and one against the hubcaps that prevents the wheels from turning. You have produced three kinds of antibodies that are specific to the car, but only the hubcap antibodies will slow the car down. Your immune system will remember how to produce all three, and doesn’t distinguish between them.
Now the virus-car mutates. It changes the changes the shape of the hubcaps, changes the material, or removes them altogether. Your immune system will remember the car – but not the hubcaps. The system doesn’t know that targeting the hubcap was the only important part, so it will ramp up its attack on the hood and bumper – minimizing the importance of all other responses. It may “tweak” its hubcap response, or perhaps even develop a new one from scratch, but that process will be slow and certainly of lower priority.
In ignoring the new hubcap response, the immune system’s memory of the original car is not only obsolete, but actively interfering with the response necessary to target the new car’s wheels. This is what immunologists call ‘original antigenic sin’ – ineffective immune memory that hampers desired responses to new pathogen strains. This phenomenon is well documented in influenza where seasonal variants and repeat vaccinations dominate the landscape. However, this sort of interference is extraordinarily difficult to quantify making it hard to routinely study.
Scientists and public health officials cannot ignore this threat in COVID-19, and must get out front of the virus. Fortunately, there is a path forward.
[Get the best of The Conversation, every weekend. Sign up for our weekly newsletter.]
Multiple-strain vaccinations offer hope
To combat this problem, significant efforts are being made to prioritize the pursuit of a single-shot flu vaccine, or a universal vaccine. The goal is to make a vaccine capable of neutralizing many different viral strains at once.
To this end, researchers have begun making headway in the development and use of complex multi-strain vaccines, capitalizing on emerging research showing that if your immune system is presented with multiple versions of the same pathogen, it will tend to choose targets that are shared between them.
Presented with a Model-T, Ford F150, and electric Mustang all at once, your immune system will often choose to ignore differences between the targets. Instead of focusing on the hood, or even the easily modified hubcaps, your immune system might recognize the shape and rubber on the tires. This altered response would not only interfere with the function of all three vehicles, but it would be targeting a region of the vehicle that is generalized. You have not created a vaccine against Mustangs, you have created a vaccine against road-based vehicles that use tires.
The recent knowledge gains in influenza vaccination must be immediately applied to SARS-CoV-2. I am hopeful that the current class of mRNA vaccines will continue to provide protection against emerging strains, but this pandemic has taught us that hoping is not enough.
Over the last year, governments around the world have stepped up to provide resources into the basic investigation of immune responses to COVID-19, and ongoing vaccination efforts. They had the foresight and courage to fund a new mRNA-based vaccination technology that has ushered in a new era in vaccination. Let’s build on that momentum and prioritize research into truly innovative approaches to vaccination that stand to benefit billions of people across the globe.
Matthew Woodruff, Instructor, Lowance Center for Human Immunology, Emory University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Fact sheet – Viral vector vaccine (J&J) overview
Singe-page overview of viral vector vaccines against SARS-CoV-2. Free to download and distribute.
Find this useful? Please consider donating to help us build more!
Not quite what you need? Work with us to build something useful to your community!
Fact sheet – How do we know the vaccine is safe?
Single-page explainer on the safety testing of the SARS-CoV-2 vaccines. Free to download and distribute!
Find this useful? Please consider donating to help us build more!
Not quite what you need? Work with us to build something useful to your community!
Fact sheet – Why does the vaccine have side effects?
A single-page explainer on why the vaccines against SARS-CoV-2 have side effects even though they are safe. Free to download and distribute.
Find this useful? Please consider donating to help us build more!
Not quite what you need? Work with us to build something useful to your community!
The COVID-19 vaccine made me feel like garbage (that’s fine!)
I got my Moderna booster! And it made me feel terrible… But that’s ok! This is an explainer about what it’s like to get the COVID vaccine, how vaccines work, and why they sometimes make us feel sick even though they are safe and effective.
“An autoimmune-like antibody response is linked with severe COVID-19”
An autoimmune-like antibody response is linked with severe COVID-19
Matthew Woodruff, Emory University
In the earliest days of the pandemic, many immunologists, including me, assumed that patients who produced high quantities of antibodies early in infection would be free from disease. We were wrong.
Several months into studying COVID-19, like other scientists, I’ve come to realize the picture is far more complicated. A recent research study published by my colleagues and me adds more evidence to the idea that in some patients, preventing dysregulated immune system responses may be as important as treating the virus itself.
I am an immunologist at Emory University working under the direction of Dr. Ignacio Sanz, Emory’s chief of rheumatology. Immune dysregulation is our specialty.
Inflammation in COVID-19
A harrowing turn in the COVID-19 pandemic occurred with the realization that the immune system’s power in fighting infection was sometimes pyrrhic. In patients with severe COVID-19 infections, evidence emerged that the inflammatory process used to fight the SARS-CoV-2 virus were, in addition to fighting the virus, potentially responsible for harming the patient. Clinical studies described so-called cytokine storms in which the immune system produced an overwhelming quantity of inflammatory molecules, antibodies triggering dangerous blood clots and inflammation of multiple organ systems, including blood vessels, in COVID-recovered children. All these were warning signs that in some patients, immune responses to the SARS-CoV-2 virus, which causes COVID-19, may have tipped from healing to destructive.
Quick thinking and courageous decisions made by physicians on the front lines led to the use of steroids, medicines that dampen the immune response, early on in the course of infection of hospitalized patients. This approach has saved lives.
But it’s not yet clear what parts of the immune system physicians are dampening that is having the effect. Understanding the nature of immune dysregulation in COVID-19 could help identify patients in whom these treatments are most effective. It may even justify more targeted and powerful approaches for modulating the immune system currently reserved for autoimmune diseases.
The right antibodies take time
Antibodies are powerful weapons. Produced by white blood cells called B cells, they latch onto infectious agents like viruses and bacteria and prevent them from infecting your healthy cells. These antibody-virus aggregates unleash powerful inflammatory reactions and serve as homing beacons that allow the rest of your immune system to target the pathogens efficiently. In some circumstances, they can even kill.
Antibodies are so powerful that cases of mistaken identity – when a B cell produces antibodies that attack a person’s own cells – can lead to widespread organ damage and establish a perpetual cycle of immune self-targeting. We refer to this state of self-destruction as an autoimmune disease.
To avoid autoimmune disaster, and to ensure effective response against the invading pathogen, B cells undergo a training process. Those that respond to the virus refine their antibodies and mature, ensuring potent antibodies capable of disabling the invader. B cells that target your own tissue are destroyed.
But that maturation process takes time. Two weeks of B cell “training” during a severe infection can mean the difference between life and death. Faster antibody responses are needed. To bridge that gap, the immune system has an alternative form of B cell activation – called extrafollicular activation – that generates fast-acting antibodies that seem to bypass many of the known safety checks that accompany a more precise response.
Extrafollicular responses develop quickly, are short-lived by design and die back when the more targeted responses emerge onto the scene.
Except when they don’t.
Autoimmune-like responses in COVID-19
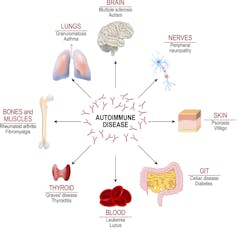
Between 2015 and 2018, our lab found that these extrafollicular immune system responses were a common characteristic of people who suffered from autoimmune diseases, such as lupus. Patients suffering from this disease show chronically active extrafollicular responses that led to high levels of self-targeted antibodies and destruction of organs such as the lungs, heart and kidneys.
The presence of specific kinds of B cells generated by extrafollicular responses in the blood can be an important indicator of disease severity in lupus, and now also COVID-19.
In a recently published paper, my colleagues and I have identified extrafollicular B cell signatures in cases of severe COVID-19 similar to those we saw in active lupus. We showed that early on in the response to infection, patients with severe disease undergo a rapid activation of this fast-track pathway for antibody production. These patients produce high levels of viral-specific antibodies, some which are capable of neutralizing the virus. However, in addition to those protective antibodies, some that we saw look suspiciously like the ones found in autoimmune disorders such lupus.
In the end, patients with these autoimmune-like B cell responses fare poorly, with high incidences of systemic organ failure and death.
Tempering immune responses in COVID-19
Let me be clear here: COVID-19 is not an autoimmune disorder. The autoimmune-like inflammatory responses my team discovered could simply reflect a “normal” response to a viral infection already out of hand.
However, even if this kind of response is “normal,” it doesn’t mean that it’s not dangerous. These prolonged extrafollicular responses have been shown to contribute to autoimmune disease severity both through the production of self-targeted antibodies and through inflammation that can damage tissue like the lung and kidney. This suggests that these early immune responses to a viral infection like COVID-19 are in tension with the later-targeted antibody response; in other words, the body’s rapid antibody production to nab the virus runs the risk of targeting not the virus, but the patient’s own organs and tissues.
[Deep knowledge, daily. Sign up for The Conversation’s newsletter.]
Immunologists like me need to learn more. Why are only some patients turning on such strong extrafollicular B cell responses? Are the antibodies that result from this response particularly prone to attacking and destroying the host’s organs? Would an ongoing autoreactive response help explain instances of “lingering” COVID-19 even after the viral infection has cleared?
Despite these uncertainties, the medical community needs to recognize that, in the appropriate patients, dampening immune responses through steroid treatment (or perhaps even more powerful autoimmune-focused therapies) is a critical weapon in combating COVID-19. Physicians and scientists must continue to build our arsenal of therapeutics around the idea that in some cases of COVID-19, controlling your response to the virus might be as important as controlling the virus itself.
Matthew Woodruff, Instructor, Lowance Center for Human Immunology, Emory University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
“COVID-19 causes some patients’ immune systems to attach their own bodies, which may contribute to severe illness”
COVID-19 causes some patients’ immune systems to attack their own bodies, which may contribute to severe illness
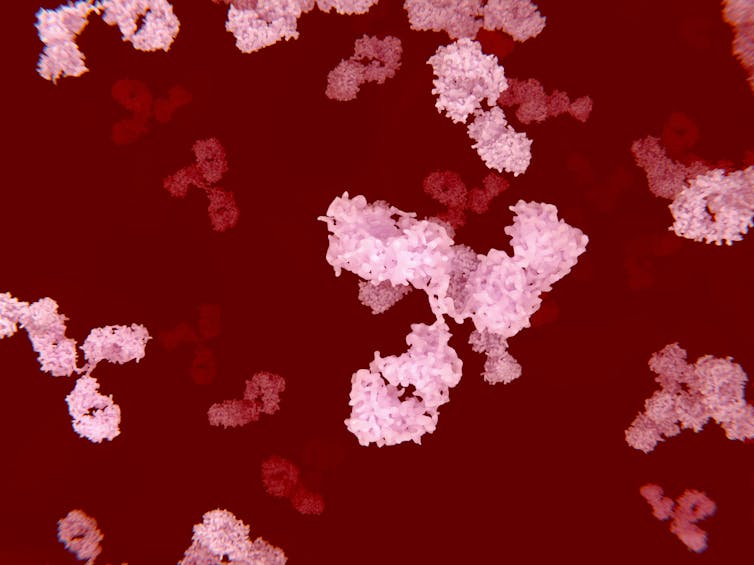
Matthew Woodruff, Emory University
Across the world, immunologists who retooled their labs to join the fight against SARS-CoV-2 are furiously trying to explain why some people get so sick while others recover unscathed. The pace is dizzying, but some clear trends have emerged.
One area of focus has been the production of antibodies – powerful proteins capable of disabling and killing invading pathogens like viruses. Of great concern has been the sporadic identification of so-called autoreactive antibodies that, instead of targeting disease causing microbes, target the tissues of individuals suffering from severe cases of COVID-19.
Early studies implicated these autoantibodies in dangerous blood clots forming in patients admitted to intensive care. More recently, they have been linked to severe disease by inactivating critical components of viral immune defenses in a significant fraction of patients with severe disease.
As an immunologist within the Lowance Center for Human Immunology at Emory University, I have been investigating the immune response responsible for producing antibodies in COVID-19. Under the direction of Dr. Ignacio Sanz, our group has previously investigated immune responses contributing to autoantibody production in autoimmune disorders like lupus, and more recently in severe cases in COVID-19. However, while we were able to characterize the response in COVID-19 patients as autoimmunelike, we could not confirm the production of autoantibodies hidden within their antiviral responses.
Now we can.
In a newly released study awaiting peer-review, we describe the alarming finding that in the sickest patients with COVID-19, autoantibody production is common – a finding with large potential impact on both acute patient care and infection recovery.
Severe infection is linked with autoantibody production
Autoantibodies come in “flavors” that are usually associated with specific disease types. Patients with lupus, for example, will often have antibodies that target their own DNA – the molecules that make up the human genome.
Patients with the autoimmune disorder rheumatoid arthritis are less likely to have those antibodies, but more likely to show positive tests for rheumatoid factor – antibodies that target other antibodies.
In this study, the Lowance Center group analyzed the medical charts of 52 patients in intensive care who were diagnosed with COVID-19. None of them had a history of autoimmune disorders. However, they were tested during infection for autoantibodies found in a variety of disorders.
The results are stark. More than half of the 52 patients tested positive for autoantibodies. In patients with the highest levels of c-reactive protein (a marker of inflammation) in the blood, more than two-thirds displayed evidence that their immune system was producing antibodies attacking their own tissue.
While these findings raise concerns, there are things that our data don’t reveal. Although patients with severe disease clearly display autoantibody responses, the data don’t tell us to what extent these autoantibodies contribute to the most severe symptoms of COVID-19.
It could be that severe viral illness routinely results in the production of autoantibodies with little consequence; this could just be the first time we’re seeing it. We also don’t know how long the autoantibodies last. Our data suggest that they are relatively stable over a few weeks. But, we need follow-up studies to understand if they are persisting routinely beyond infection recovery.
Importantly, we believe that the autoreactive responses we have identified here are specific to the SARS-CoV-2 infection – there is no reason to believe that similar results would be expected through vaccination against the virus.
Understanding the role of autoantibodies in COVID-19
However, while it is possible that these autoantibodies are benign, or even helpful in a yet-unidentified manner, it’s also possible that they aren’t. Maybe these self-targeted antibody responses do indeed contribute to disease severity, helping explain the delayed onset of severe symptoms in some patients that may correlate with antibody production.
This could be a reason that treatment with dexamethasone, an immunosuppressant often used to quell “flare-ups” of autoimmune disorders, might be effective in treating patients with only the most severe disease. It is also possible that these responses are not short lived, outlasting the infection and contributing to ongoing symptoms now experienced by a growing number of “long-hauler” COVID-19 patients.
Most concerning, it is possible that these responses could self-perpetuate in some patients, resulting in the emergence of new, permanent autoimmune disorders.
My colleagues and I sincerely hope that this is not the case – rather, that the emergence of autoantibodies in these patients is a red herring, a quirk of a viral immune response in some patients that will resolve on its own. But we need to do better than hope – we need to ask the right questions and figure out the answers. Fortunately, this study also gives us the tools to do that.
Autoreactive antibody test may reveal better treatments
The tests that were run on these patients to determine their “autoreactive profile” are not specialized. They are available to most hospital labs across the country. Indeed, the two most common antibodies that we find in these patients, antinuclear antibodies and rheumatoid factor, are detected by common tests used by rheumatologists.
Our study shows that by testing for just these two autoantibodies, and the inflammatory marker c-reactive protein, we may be able to identify patients more likely to be experiencing potentially dangerous immune responses that might benefit from more aggressive immune modulation.
[Get facts about coronavirus and the latest research. Sign up for The Conversation’s newsletter.]
Further, autoreactivity testing might help identify patients who might benefit from rheumotological follow-up to monitor recovery, and help us understand whether some cases of “long-hauler” COVID-19 might be related to persisting autoantibodies. If so, these patients might respond to the same immune-targeted therapies that have been successful in MIS-C where autoantibody production has now been documented.
Finally, by testing patients immediately following COVID-19 recovery, we can establish baselines and begin to track the possible emergence of new cases of autoimmunity following this terrible disease, and plan early rheumatological intervention if needed.
We now have the tools. It’s time to start using them.
Matthew Woodruff, Instructor, Lowance Center for Human Immunology, Emory University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
“Vaccines against SARS-CoV-2 will have side effects – that’s a good thing”
Vaccines against SARS-CoV-2 will have side effects – that’s a good thing
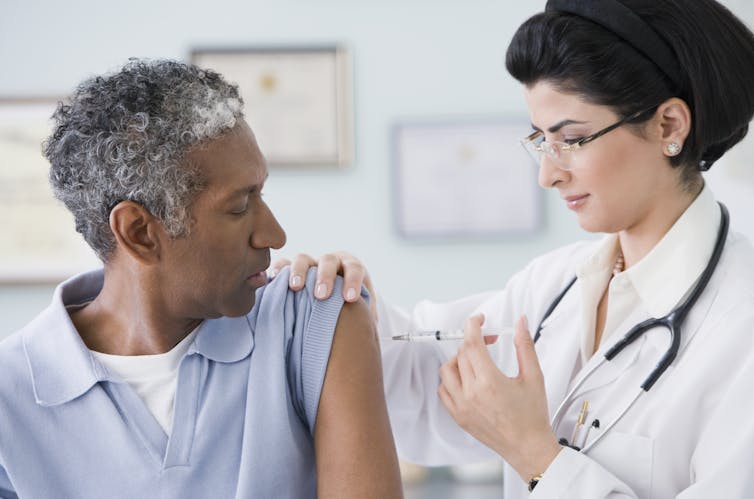
Matthew Woodruff, Emory University
Takeaways
- Temporary side effects from vaccines are a normal sign of a developing immune response.
- Vaccines work by training your immune system to recognize and remember a pathogen in a safe way.
- Expected side effects from a COVID-19 vaccine include redness and swelling at the injection site and stiffness and soreness in the muscle.
- A potent vaccine may even cause fever. It does not mean that the vaccine gave you COVID-19.
In 2021 hundreds of millions of people will be vaccinated against SARS-CoV-2. The success of that COVID-19 vaccination campaign will heavily depend on public trust that the vaccines are not only effective, but also safe. To build that trust, the medical and scientific communities have a responsibility to engage in difficult discussions with the public about the significant fraction of people who will experience temporary side effects from these vaccines.
I am an immunologist who studies the fundamentals of immune responses to vaccination, so part of that responsibility falls on me.
Simply put, receiving these vaccines will likely make a whole lot of people feel crappy for a few days. That’s probably a good thing, and it’s a far better prospect than long-term illness or death.
Immunology’s ‘dirty little secret’
In 1989, immunologist Charles Janeway published an article summarizing the state of the field of immunology. Until that point, immunologists had accepted that immune responses were initiated when encountering something foreign – bacteria, viruses, and parasites – that was “non-self.”
Janeway suspected that there was more to the story, and famously laid out what he referred to as “the immunologist’s dirty little secret”: Your immune system doesn’t just respond just to foreign things. It responds to foreign things that it perceives to be dangerous.
Now, 30 years later, immunologists know that your immune system uses a complex set of sensors to understand not only whether or not something is foreign, but also what kind of threat, if any, a microbe might pose. It can tell the difference between viruses – like SARS-CoV-2 – and parasites, like tapeworms, and activate specialized arms of your immune system to deal with those specific threats accordingly. It can even monitor the level of tissue damage caused by an invader, and ramp up your immune response to match.
Sensing the type of threat posed by a microbe, and the level of intensity of that threat, allows your immune system to select the right set of responses, wield them precisely, and avoid the very real danger of immune overreaction.
Vaccine adjuvants bring the danger we need
Vaccines work by introducing a safe version of a pathogen to a patient’s immune system. Your immune system remembers its past encounters and responds more efficiently if it sees the same pathogen again. However, it generates memory only if the vaccine packs enough danger signals to kick off a solid immune response.
As a result, your immune system’s need to sense danger before responding is at once extremely important (imagine if it started attacking the thousands of species of friendly bacteria in your gut!) and highly problematic. The requirement for danger means that your immune system is programmed not to respond unless a clear threat is identified. It also means that if I’m developing a vaccine, I have to convince your immune system that the vaccine itself is a threat worth taking seriously.
This can be accomplished in a number of ways. One is to inject a weakened – what immunologists call attenuated – or even killed version of a pathogen. This approach has the benefit of looking almost identical to the “real” pathogen, triggering many of the same danger signals and often resulting in strong, long-term immunity, as is seen in polio vaccination. It can also be risky – if you haven’t weakened the pathogen enough and roll out the vaccine too fast, there is a possibility of unintentionally infecting a large number of vaccine recipients. In addition to this unacceptable human cost, the resulting loss of trust in vaccines could lead to additional suffering as fewer people take other, safer vaccines.
A safer approach is to use individual components of the pathogen, harmless by themselves but capable of training your immune system to recognize the real thing. However, these pieces of the pathogen don’t often contain the danger signals necessary to stimulate a strong memory response. As a result, they need to be supplemented with synthetic danger signals, which immunologists refer to as “adjuvants.”
Adjuvants are safe, but designed to inflame
To make vaccines more effective, whole labs have been dedicated to the testing and development of new adjuvants. All are designed with the same basic purpose – to kick the immune system into action in a way that maximizes the effectiveness and longevity of the response. In doing so, we maximize the number of people that will benefit from the vaccine and the length of time those people are protected.
To do this, we take advantage of the same sensors that your immune system uses to sense damage in an active infection. That means that while they will stimulate an effective immune response, they will do so by producing temporary inflammatory effects. At a cellular level, the vaccine triggers inflammation at the injection site. Blood vessels in the area become a little more “leaky” to help recruit immune cells into the muscle tissue, causing the area to become red and swell. All of this kicks off a full-blown immune response in a lymph node somewhere nearby that will play out over the course of weeks.
In terms of symptoms, this can result in redness and swelling at the injection site, stiffness and soreness in the muscle, tenderness and swelling of the local lymph nodes and, if the vaccine is potent enough, even fever (and that associated generally crappy feeling).
This is the balance of vaccine design – maximizing protection and benefits while minimizing their uncomfortable, but necessary, side effects. That’s not to say that serious side effects don’t occur – they do – but they are exceedingly rare. Two of the most discussed serious side effects, anaphalaxis (a severe allergic reaction) and Guillain-Barré Syndrome (nerve damage due to inflammation), occur at a frequency of less than 1 in 500,000 doses. https://www.youtube.com/embed/kmfZJvvkVhY?wmode=transparent&start=0 Side effects are normal.
Vaccination against SARS-CoV-2
Early data suggest that the mRNA vaccines in development against SARS-CoV-2 are highly effective – upwards of 90%. That means they are capable of stimulating robust immune responses, complete with sufficient danger signaling, in greater than nine out of 10 patients. That’s a high number under any circumstances, and suggests that these vaccines are potent.
So let’s be clear here. You should expect to feel sore at the injection site the day after you get vaccinated. You should expect some redness and swelling, and you might even expect to feel generally run down for a day or two post-vaccination. All of these things are normal, anticipated and even intended.
While the data aren’t finalized, more than 2% of the Moderna vaccine recipients experienced what they categorized as severe temporary side effects such as fatigue and headache. The percentage of people who experience any side effects will be higher. These are signs that the vaccine is doing what it was designed to do – train your immune system to respond against something it might otherwise ignore so that you’ll be protected later. It does not mean that the vaccine gave you COVID-19.
[Deep knowledge, daily. Sign up for The Conversation’s newsletter.]
It all comes down to this: Some time in the coming months, you will be given a simple choice to protect yourself, your loved ones and your community from a highly transmissible and deadly disease that results in long-term health consequences for a significant number of otherwise healthy people. It may cost you a few days of feeling sick.
Matthew Woodruff, Instructor, Lowance Center for Human Immunology, Emory University
This article is republished from The Conversation under a Creative Commons license. Read the original article.